- SMT labelling machines
- Multi-station labelling machines
- High precision labelling machines
- PCB labelling machines
- Pallet labelling machines
- Automatic labelling machines
- High-speed labelling machines
- Double-sided labeling machine
- Self-adhesive labeling machine
- FPC adhesive backing machine
- Mobile phone adhesive backing machine
- speaker sticker backing machine
- Automatic backing machine
- Automatic adhesive backing machine
- Search
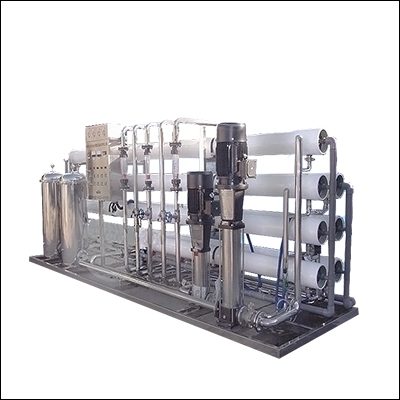
Medical ultrapure water equipment

- Product:Medical ultrapure water equipment
- Catalogue:Labelling machines
- Views:177627times
- Update:2018/10/10 9:16:42
- Call:133-6065-4218
Medical ultra-pure water equipment process:
Raw water -- raw water pressure pump -- multi-medium filter -- activated carbon filter -- soft water filter -- precision filter -- first-stage reverse osmosis machine -- deaeration film -- intermediate water tank -- intermediate water pump -- EDI system -- purification water tank -- pure water pump -- ultraviolet sterilizer -- microporous filter -- pasteurization -- water point (new process of Chinese pharmacopoeia, 2005)
Raw water, raw water pump - medium filter - activated carbon filter - water softener - precision filters - the first level of reverse osmosis - PH adjustment - the middle water tank - 2 RO (reverse osmosis membrane surface is positively charged) - RO water tank - RO water pump - - TOC resolver - - EDI systems - purification tank - pure water pump - uv sterilizer - microporous filters - pasteurized - water point (Chinese pharmacopoeia water for injection, the European Union and the United States pharmacopoeia recommended process)
Raw water - raw water pressurized pump - multi-medium filter - activated carbon filter - soft water filter - precision filter - first-stage reverse osmosis equipment - intermediate water tank - intermediate water pump - ion exchanger - purification water tank - pure water pump - ultraviolet sterilizer - microporous filter - water point (2000 edition Chinese pharmacopoeia traditional process)
Raw water, raw water pump - medium filter - activated carbon filter - water softener - precision filters - the first level of reverse osmosis - PH adjustment - the middle water tank - 2 ro (reverse osmosis membrane surface is positively charged) - purification tank - pure water pump - uv sterilizer - microporous filter - pasteurized - water points (2005 edition of China pharmacopoeia currently circulating process)
Purified aquatic water index:
Chemical indicators: meet the requirements of pharmacopoeia of the People's Republic of China 2005 edition of pharmaceutical purified water
Hygiene examination: microbe 10CFU/100ml
Endotoxin 0.25 EU/ml
Conductivity 2 mu S/cm or less (resistivity acuity 0.5 M Ω * cm)
Basic technical characteristics of equipment:
The system adopts automatic control (manual control is also available), and automatic backwashing and regeneration procedures can be set when the system is running.
The primary reverse osmosis and secondary reverse osmosis are provided with backflow pipeline, and the reverse osmosis equipment is provided with chemical cleaning device and disinfection device.
There is a PH adjustment device between the first reverse osmosis and the second reverse osmosis to ensure that the water conductivity of the equipment meets the requirements of pharmacopoeia.
The secondary reverse osmosis membrane adopts the anti-pollution reverse osmosis membrane with positive charge to ensure the long-term stable operation of reverse osmosis equipment.
The primary reverse osmosis pipeline is made of 304 stainless steel, and the secondary reverse osmosis is made of 316L stainless steel.
In the first stage reverse osmosis and the second stage reverse osmosis equipment are equipped with online conductance detection instrument, the conductivity of water production can be viewed at any time.
Low voltage protection switch is set before the first reverse osmosis, and low voltage protection switch and high voltage protection switch are set before the second reverse osmosis.
The water recovery rate of primary and secondary reverse osmosis can be adjusted, the recovery rate of primary reverse osmosis is 60-65%, and the recovery rate of secondary reverse osmosis is 70%.
The pre-treatment device adopts the original imported parts; UPVC pipes are used for piping between pretreatment equipment.
The purified water storage tank is equipped with respiratory filter, and the conveying pipeline is equipped with ultraviolet sterilizer and microporous filter to ensure that the pure water meets the sanitary requirements.
Equipment advantages:
1, the structural design should be simple, reliable, easy to disassemble and assemble.
2, in order to facilitate the disassembly, replacement, cleaning parts, the implementation of the design of the mechanism to use standardized, universal, systematic parts.
3. The inner and outer wall surface of the equipment should be smooth and smooth, without dead Angle, easy to clean and sterilize. The surface of parts shall be treated with chrome plating to resist corrosion and prevent rust. Avoid using paint on the outside of the equipment to prevent peeling.
4. The equipment for preparing purified water shall use low-carbon stainless steel or other materials that have not been proven to pollute the water quality. Equipment preparing purified water should be cleaned regularly and the cleaning effect verified.
5. The materials in contact with wsi must be high quality low-carbon stainless steel (e.g. 316L stainless steel) or other materials that have not been proven to contaminate the water quality. The equipment for preparing water for injection should be cleaned regularly and the cleaning effect verified.
6, purified water storage cycle should not be more than 24 hours, the storage tank should be made of stainless steel materials or proven non-toxic, corrosion resistant, do not exudate contaminated ions other materials.
7. The pipes and pumps for transporting purified water and water for injection shall be cleaned, disinfected and sterilized regularly, and can be put into use after verification.